

The nuclear binding energy varies between nuclei.This increases the mass of nuclei with more neutrons than protons relative to the atomic mass unit scale based on 12C with equal numbers of protons and neutrons. The neutron is slightly heavier than the proton.There are two reasons for the difference between mass number and isotopic mass, known as the mass defect: For example, 63Cu (29 protons and 34 neutrons) has a mass number of 63, and an isotopic mass in its nuclear ground state is 62.91367 u. The isotopic mass usually differs for other isotopes and is usually within 0.1 u of the mass number. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.įor 12C, the atomic mass is exactly 12u since the atomic mass unit is defined from it. One atomic mass unit is equal to 1.66 x 10 -24 grams.
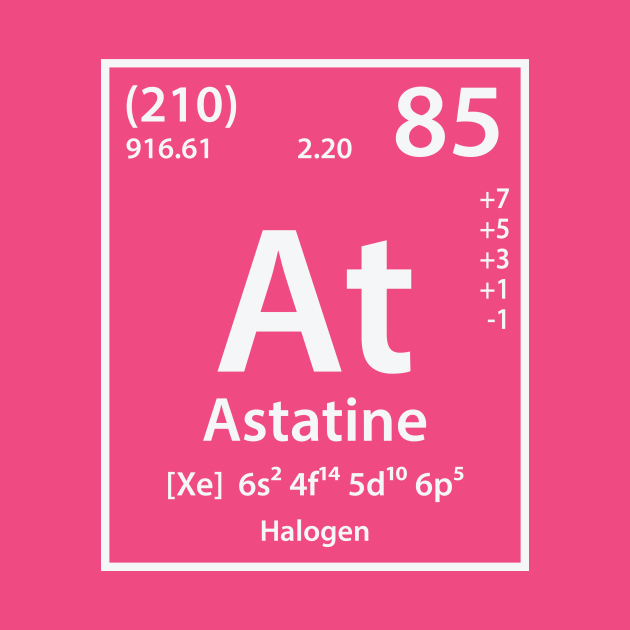
The unit of measure for mass is the atomic mass unit (amu). Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. See also: Properties of Astatine Atomic Mass of Astatine Thermal properties Melting Point 302 Boiling Point 337 Thermal Conductivity 2 Specific Heat - Heat of Fusion - Heat of Vaporization. The bulk properties of astatine are not known with any certainty.Īstatine – Properties Element Astatine Atomic Number 85 Symbol At Element Category Metalloids Phase at STP Solid Atomic Mass 210 Density at STP - Electron Configuration 6p5 Possible Oxidation States Electron Affinity 270.1 Electronegativity 2.2 1st Ionization Energy 9.5 Year of Discovery 1940 Discoverer Corson, Dale R. It occurs on Earth as the decay product of various heavier elements. The chemical symbol for Astatine is At.Īstatine is the rarest naturally occurring element on the Earth’s crust. Therefore, there are various non-equivalent definitions of atomic radius.Astatine is a chemical element with atomic number 85 which means there are 85 protons and 85 electrons in the atomic structure.
ASTATINE SYMBOL FREE
However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. It must be noted, atoms lack a well-defined outer boundary. The atomic radius of Astatine atom is 150pm (covalent radius). Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. Mass numbers of typical isotopes of Astatine are 210. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z.įor stable elements, there is usually a variety of stable isotopes. Neutron number plus atomic number equals atomic mass number: N+Z=A. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Atomic Number – Protons, Electrons and Neutrons in AstatineĪstatine is a chemical element with atomic number 85 which means there are 85 protons in its nucleus.


 0 kommentar(er)
0 kommentar(er)
